

Our adherence to global standards and and data integrity
At Chemo Test Laboratory, we foster a positive, results-driven work environment that offers continuous learning opportunities at every stage. Our workplace culture is built on collaboration, professional growth, and innovation, ensuring that every team member can learn, evolve, and thrive. We take pride in our supportive and inclusive work atmosphere, where colleagues uplift each other, leaders inspire and motivate, and a secure work environment allows individuals to excel with confidence.
We take pride in our supportive and inclusive work atmosphere, where colleagues uplift each other, leaders inspire and motivate, and a secure work environment allows individuals to excel with confidence.

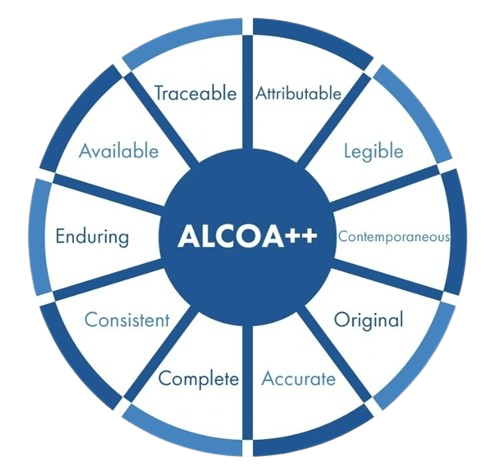
At Chemo Test Laboratory, data integrity is at the core of our quality commitment. We rigorously follow the ALCOA++ framework. This structured approach enables us to maintain the highest standards of reliability and regulatory compliance across every stage of testing and reporting
To maintain data integrity and ensure regulatory compliance, Chemo Test Laboratory implements a comprehensive user access management system. Each user is assigned specific roles and privileges based on their responsibilities, with strict controls over login credentials, role assignment, and password policies. This structured approach supports traceability, accountability, and adherence to 21 CFR Part 11 guidelines.

Distinct login credentials are provided for each user category
21 CFR regulations compliant
Administrator; QA Reviewer; Technical Reviewer; QC Reviewer; User/Analyst
Only Administrators have the authority to add or delete roles and privileges
Clear definitions of rights and privileges for each user group
Updated every 90 days for security purposes